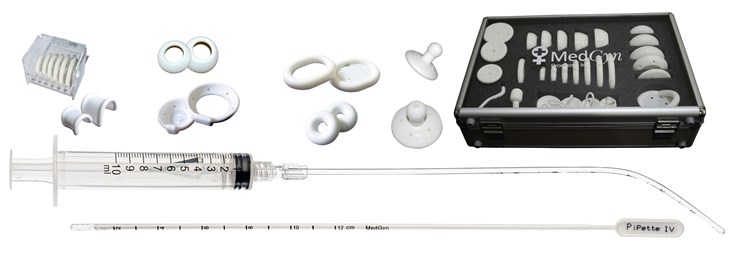
Medgyn specialises in providing unique, high-quality medical devices for obstetrics and gynaecology professionals throughout the world. The product lines include sterile disposable medical devices (single use), diagnostic test kits and various OB/GYN procedure kits. These are used in physician offices, multi-practice clinics and hospitals.
Some of the most popular products that we sell currently are the endosamplers, pipettes, pessaries and speculums. We can also supply anything else from the MedGyn catalogue.
****All pessaries are now available via NHS Supply Chain****
We have some offers available on MedGyn products, please speak to our friendly office or contact your local representative.
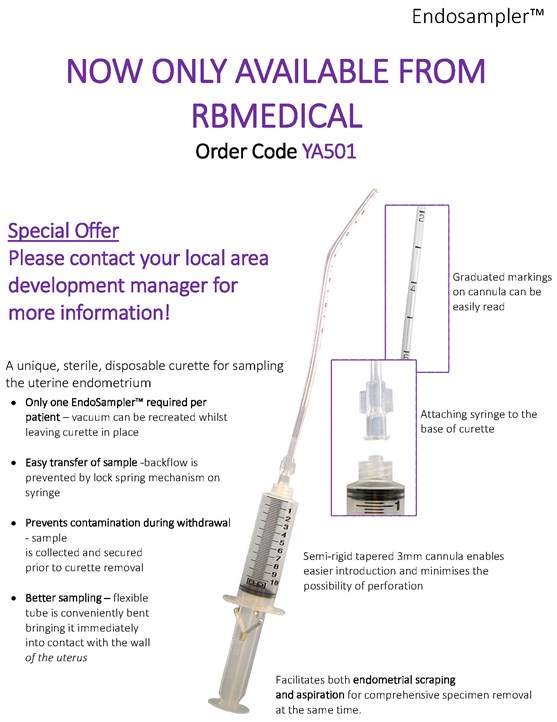
MedGyn Endosampler
You can view a video of the endosampler in use by clicking this link
ENDOSAMPLER I AND II
DEVICE DESCRIPTION:
MedGyn Endosampler is a sterile, disposable catheter/cannula with a 10 ml syringe that is fitted with a locking clip. The semi-rigid cannula is 3 mm in diameter with graduated markings and is bent conveniently with a single or double slot for sampling from all areas of the endometrial cavity including the cornual areas.
INDICATIONS FOR USE:
-
The device is meant for uterine diagnostic and therapeutic uses.
-
The device is used for obtaining clearly differentiated endometrial tissue sample without anesthesia in an outpatient setting or as in an office setting.
-
The sample is used for cytologic and histologic diagnosis.
CONTRAINDICATIONS:
CURETTAGE SHOULD NOT BE PERFORMED FOR:
-
Patients where pregnancy is suspected unless a termination of pregnancy is to be carried out.
-
Patients with or recently recovered from pelvic inflammatory disease.
WARNINGS:
-
STERILE unless package opened or damaged
-
SINGLE USE ONLY. DO NOT REUSE.
-
DISPOSABLE - DISCARD AFTER USE.
-
STERILIZED BY ETHYLENE OXIDE
CAUTION:
Federal law restricts this device to sale by or on the order of a physician.
PRECAUTIONS:
• This procedure is to be used by a physician who has been trained thoroughly in operative obstetrics and gynecology.
• All instructions to be carefully read prior to the use of the vacuum aspiration curette/catheter.
• Pelvic examination should be accomplished to determine size and position of the uterus.
• A thorough speculum examination of the vaginal canal is to be accomplished and indicated smears of cultures are to be obtained prior to sounding.
• Care should be exercised to avoid the possibility of uterine perforation as well as cervical lacerations with the use of any aspiration catheter, sound, canal finder, fundus locator or dilator.
• Do not re-use the Endosampler. If the device is reused it can cause a cross infection or may have loss of function.
ADVERSE REACTIONS:
• Any cervical manipulation may cause a reaction. Patient should be watched for unusual weakness or nausea.
• In rare cases there may be mild cramp or spotting. If bleeding persists, patient should be instructed to contact her physician.
PRODUCTS:

INSTRUCTIONS FOR USE:
-
Prepare the vaginal area and cervix for a sterile intrauterine procedure using an antiseptic solution.
-
With a vaginal speculum in place, carefully examine the depth of uterine cavity. It may be necessary to use a tenaculum to grasp the cervix to straighten the cervical canal.
-
Gently insert the curette. If cervical canal is very dry, apply a small amount of sterile water-soluble gel to the tip of curette.
-
Attach syringe to the base of curette.
-
Withdraw the plunger fully of lock the clip in place to create a vacuum in
the curette.
-
Use long slow strokes to obtain sample from the four quadrants of
the endometrium. If the syringe fills with tissue and needs additional vacuum, disconnect syringe leaving the curette in place. Unlock the spring mechanism and empty the contents into a container with fixative. Reattach the syringe to curette and continue.
-
When curettage is completed, withdraw the curette from uterus and expel tissue into the container with fixative. Endometrial tissue is hygroscopic and should be placed in fixative to maintain the tissue in a satisfactory condition for the pathologist.
-
Withdraw fixative into curette and syringe and flush into container to wash out any tissue that may have lodged in curette.
-
Allow patient to remain in the same position for about 10 minutes.
MedGyn Cryotherapy System
Product Code LA500

The MedGyn Cryotherapy System was designed by physicians for safety, ease-of-use and versatility for all cryogenic procedures.
Light-weight, perfectly balanced and comfortable, preventing fatigue of hand and fingers during procedures.
Its superior cryo technology combined with 3-position trigger delivers optimal cryosurgical temperatures.
A wide variety of autoclavable tips is available. Remember to specify Nitrous Oxide (N2O) or Carbon Dioxide (CO2) gas and tank size.
- Single-hand control from three-position trigger (freeze, off, defrost)
- Instant defrost
- Trigger position for immediate “active” defrost process
- Extensive choice of autoclavable tips
- Capture “O” ring design provides better gas seals where tips attach to probe system
- Valve body designed and manufactured for long, trouble-free life
- Built-in regulators control pressure at tips for added safety and gas economy
- Nitrous Oxide (N2O) and Carbon Dioxide (CO2) option
- 6 lb. and 20 lb. cylinder options
MedGyn Pipette IV (Four Holes) Endometrial Sampler
YA505
MedGyn Pipette IV is used for obtaining endometrial tissue in the outpatient setting. It is designed to improve ease of tissue collection and to reduce the physical discomfort to the patient.
- Polypropylene sheath 3.1 mm external diameter
- 2.6mm internal diameter
- 23.5cm long
- 4 lateral orifices - 2.5mm in diameter
- 11 guide marks

Features
- Sterile, single use, latex free
- Semi-rigid, tapered 3.1mm cannula enables easier insertion and minimises the possibility of perforation
- Four holes in distal end for improved sample collection of the uterine cavity
- Easier dispensing of histology specimen - plunger slides freely inside cannula and is easy to push even when fully extended
- Flexible cannula offers better sampling from all areas of the endometrial cavity including difficult to reach
cornual areas - Can be manipulated to retain a fixed curved shape
- Stop on proximal end of cannula prevents plunger overrunning and jamming at distal end Ergonomically designed thumb plate
INTRAUTERINE DEVICE (IUD) RETRIEVER
The MedGyn IUD Retriever is designed to quickly and easily locate lost IUD threads and can also be used to sound the uterus for the presence of the intrauterine device. This sterile, single use device contains everything needed to insert or remove an intrauterine device.
Seven specifically designed notches at 10mm intervals (on either side) combined with a long handle (240mm), allow for greater control and optimum retrieval of IUD threads.
INDICATIONS FOR USE:
This is a sterile, single use device needed to remove an intrauterine device.
CONTRAINDICATIONS:
-
The IUD Retriever should not be used in patients with a known hypersensitivity to any of the materials that make up the composition of the device.
-
The IUD Retriever should not be used in patients with chronic cervical infections.
WARNINGS:
-
Contents are only considered sterile in unopened and undamaged sterilized packaging.
-
This device is meant for single-use only. Do not reuse this device. Dispose of the device after use.
-
This device is sterilized by ethylene oxide.
PRECAUTIONS:
-
This device is to be used by a physician thoroughly trained in operative obstetrics and gynecology.
-
All instructions are to be carefully read prior to use.
-
Do not re-use this device. If the device is reused it can cause
a cross infection or may have loss of function.
ADVERSE REACTIONS:
Adverse reactions include occasional transient uterine cramping and minimal uterine bleeding.
INSTRUCTIONS FOR USE:
-
Removal of intrauterine contraceptive devices (IUC) is typically an uncomplicated procedure, requiring simply grasping the IUC strings and pulling gently [2,3]. Missing IUC strings that are not visible at the external cervical os is a commonly encountered complication of IUC removal and use.
-
If threads are not visible, perform an ultrasound examination to confirm location and possible perforation.
-
Consider using a tenaculum to mobilize the cervix to allow better visualization as well as dilation to expand the visual field.
CAUTION:
Federal law restricts this device to sale by the order of or for use by a physician.
PESSARY:
Instructions for use:
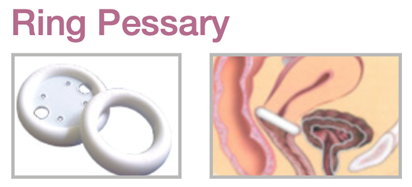
Device Description: Ring Pessary is ring-shaped with a solid core and an indentation on each side. The Ring Pessary size ranges from 51mm to 102mm and is available with support and without support.
INDICATIONS FOR USE:
The Ring Pessary is used for mild first-degree prolapse. Ring Pessary with support can also be used for an accompanying cystocele.
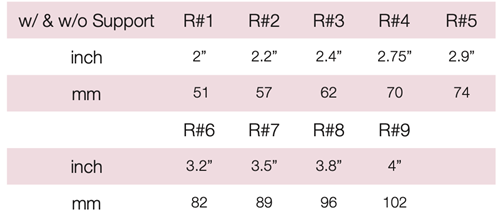

Device Description: Pessary Ring with Knob is shaped like a ring with knob on one side of the device. The device diameter ranges from 57mm to 89mm. These pessaries are available in Ring with Knob with support and without support.
INDICATIONS FOR USE:
This Pessary is indicated for patients with a mild cystocele accompanying a mild prolapse as well as urinary incontinence.
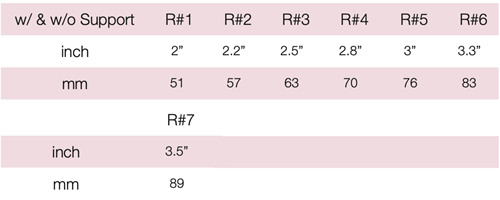
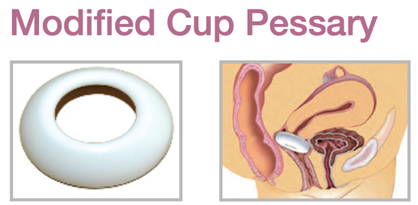
Device Description: Modified Cup Pessary is shaped like a bowl and is indicated for mild prolapse. The Pessary comes in six sizes with outer diameters 65mm or 70mm, and inner diameter of 32mm or 35mm.
INDICATIONS FOR USE:
The Modified Cup Pessary is indicated for mild prolapse.
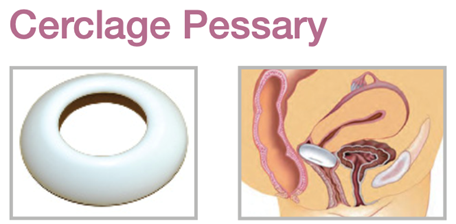
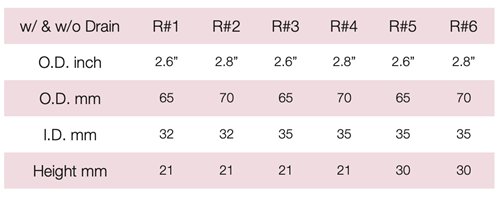
Device Description: Cerclage Pessary is shaped like a bowl and is indicated for mild prolapse. The Pessary comes in six sizes with outer diameters 65mm or 70mm, and inner diameter of 32mm or 35mm.
INDICATIONS FOR USE:
The Cerclage= Pessary is indicated for mild prolapse.
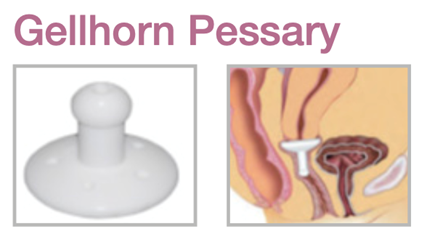
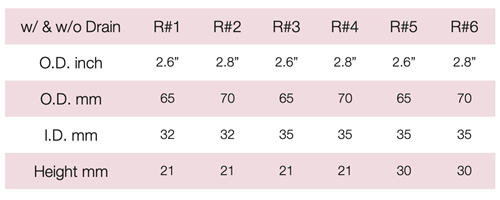
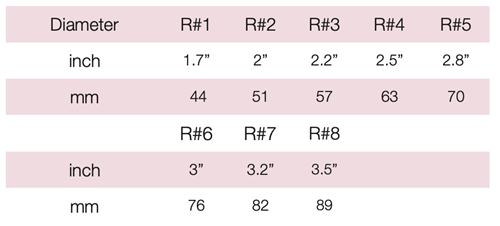
Device Description: Gellhorn Pessary is indicated for effective support for cystocele and rectocele. The Gellhorn pessary with drain available in sizes ranging from 44mm to 82mm.
INDICATIONS FOR USE:
The Gellhorn Pessary is used for second- to third-degree prolapse or procidentia.
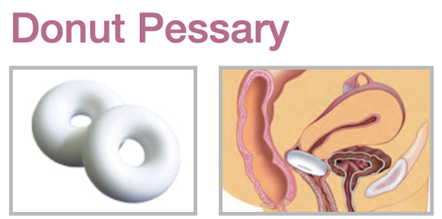
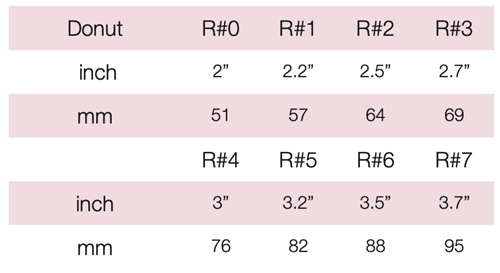
Device Description: Donut Pessary is shaped like a donut and the interior of the donut is hallow. The Donut Pessary is available in sizes ranging from 51mm to 95mm.
INDICATIONS FOR USE:
The Donut Pessary is used for second- to third-degree prolapse as well as cystocele.
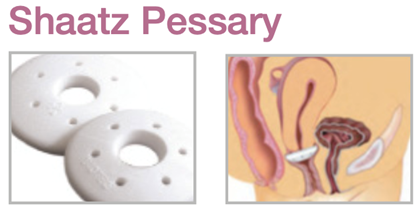
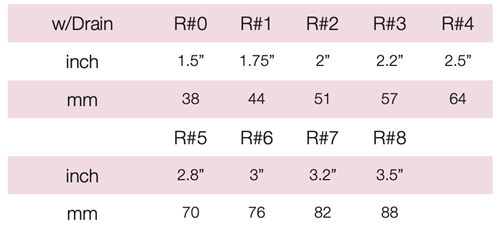
Device Description: Shaatz Pessary is circular in shape with a center hole. Shaatz pessaries with drain are available in sizes ranging from 38mm to 88mm.
INDICATIONS FOR USE :
The Shaatz Pessary is indicated for first- to second-degree prolapse as well as cystocele.
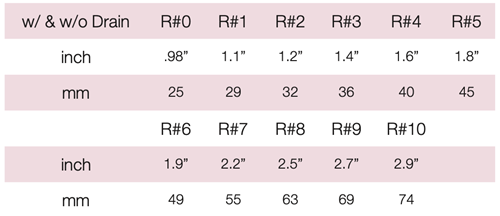
Device Description: Cube Pessary is shaped like a cube with rounded corners. Each side has a circular suction facet. These Cube Pessaries are available in sizes ranging from 25mm to 74mm. The Cube pessary is available both with or without drainage holes and has a silicone tie to aid in removal.
INDICATIONS FOR USE:
The Cube Pessary is indicated for third-degree prolapse, including procidentia, as well as a cystocele and a rectocele.
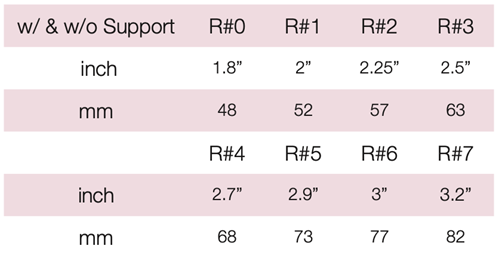
Device Description: Cup Pessary is shaped like a cup with rounded corners. These Cup Pessaries are available of sizes ranging from 48mm to 82mm.
INDICATIONS FOR USE:
The Cup Pessary is used for first- or a mild second-degree uterine prolapse.
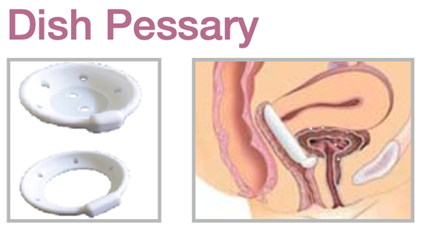
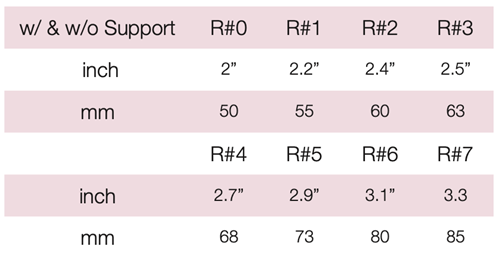
Device Description: Dish Pessary is shaped like a dish to provide bladder neck support to relieve stress incontinence and minor degrees of prolapse. The Dish Pessaries are available sizes from 50mm to 85mm.
INDICATIONS FOR USE:
The Dish Pessary is used for first- or second-degree uterine prolapse as well as cystocele. This pessary may also relieve urinary incontinence.
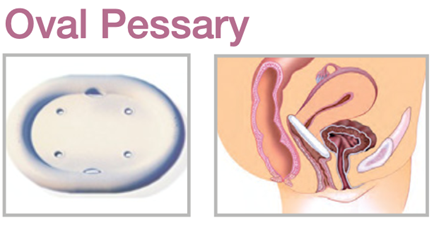
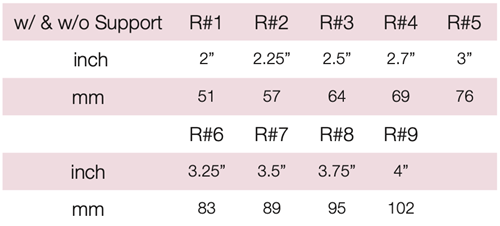
Device Description: Oval Pessary is designed specifically to
fit a narrow vaginal vault. These Oval Pessaries are available in sizes from 51mm to 102mm.
INDICATIONS FOR USE:
The Oval Pessary is used for first- to second-degree prolapse. Oval Pessary can also be used for an accompanying cystocele.
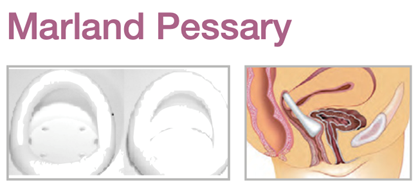
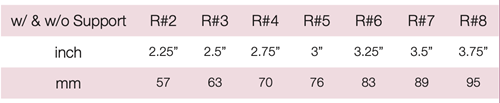
Device Description: Marland Pessary is for moderate to extensive cystocele, as well as providing support for the bladder neck in managing stress incontinence. The Marland Pessaries are available in sizes ranging from 57mm to 95mm.
INDICATIONS FOR USE:
The Marland Pessary is available with or without supporting membrane, is used to relieve the symptoms of a second or third-degree uterine prolapse, a cystocele or a rectocele.
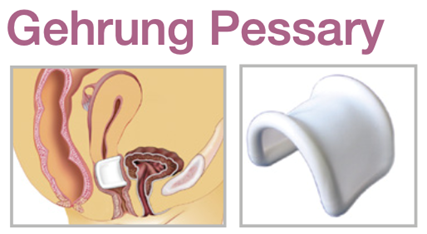
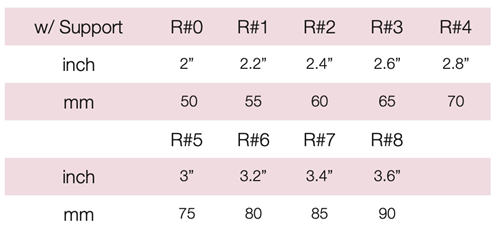
Device Description: MedGyn Gehrung knob with support is for effective support for cystocele and rectocele. They are also more adaptable in cases of procidentia where the uterus tends to herniate when other pessaries are used. The Gehrung Pessaries are made of sizes ranging from 50mm to 90mm.
INDICATIONS FOR USE:
The malleable Gehrung pessary with support membrane is primarily used for a significant cystocele or rectocele and may also provide relief for second or third-degree uterine prolapse.
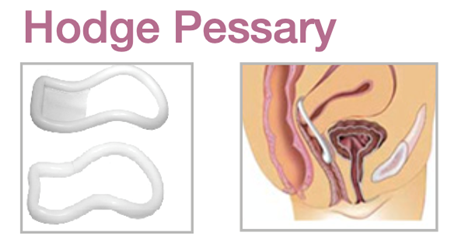
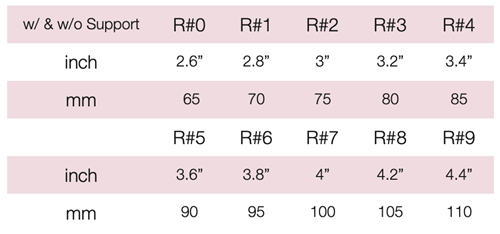
Device Description: Hodge Pessary is designed for stress urinary incontinence and for women with a small vaginal introitus. The Hodge Pessary is made sizes ranging from 65mm to 110mm. Hodge Pessary comes in with or without support.
INDICATIONS FOR USE:
The Hodge Pessary is commonly used for a first or mild second-degree uterine prolapse and may also relieve a cystocele. A Hodge Pessary with support is also effective in the prevention of exercise incontinence.
PREPARATION AND PLACEMENT:
1. This procedure is to be performed by a physician trained thoroughly in obstetrics and gynecology.
2. All instructions to be carefully read prior to use.
3. Please clean before use.
4. Instruct patient to empty her bladder.
5. Wear dry gloves.
6. Lubricate entering end of Pessary with tap water, if necessary.
7. Use finger to depress perineum.
8. Compress and guide the Pessary:
For Shaatz- Once the large flat disc is inside the vagina, push pessary upward untill resting on cervix; For Gellhorn- Once the large flat disc is past the introitus, push pessary upward until end of the stem shows; For Dish- Direct the entering end of pessary (opposite side of knob) past cervix into the posterior fornix; For Donut, Dish, Cube, Cerclage, Modified Cup Pessary, Oval, Ring and Ring with Knob-Allow Pessary to assume shape after it passes introitus.
9. Posteriorly guide Pessary rim around cervix and into the posterior fornix.
10. Use the examining finger to turn Pessary one quarter turn so its folding axis is no longer parallel with vaginal canal. This will help prevent Pessary from folding and moving. 11. The patient should be examined 24-48 hours after Pessary insertion.
CONTRAINDICATIONS:
The use of the Pessary is contraindicated with the following:
1. Non compliant Patient
2. Vaginal abrasions
3. Pelvic Infections/ Pelvic inflammatory disease/Genitourinary tract infections 4. Endometriosis
5. Sexual intercourse is contraindicated with Pessary in place.
WARNINGS: The device should be used only if package is dry, and unopened.
CAUTION:
Federal law restricts this device to sale on the order of or for use by a physician.
PRECAUTIONS:
1. This device is to be used by a physician thoroughly trained in operative obstetrics and gynecology.
2. All instructions for the device are to be carefully read prior to its use.
3. Wash hands before handling the Pessary.
ADVERSE REACTIONS:
1. While mild and/or transient symptoms appear to be fairly common during Pessary use, rare serious adverse effects may also occur, particularly if Pessary care is neglected.
2. Serious adverse events related to Pessary use include impaction or entrapment of the Pessary requiring surgical removal and infection and/or obstruction.
RECOMMENDED CLEANING INSTRUCTIONS:
1. Prepare a cleaning solution by mixing a mild soap (such as Dawn or equivalent) with tap water using the soap’s manufacturer’s recommended concentration. Prepare this solution in a container large enough to fully submerge the device.
2. Soak and Scrub
a. Soak the device in the container of prepared soap solution for a minimum of 5 minutes.
b. Following the 5-minute soak period, scrub the device for a minimum of 15 seconds with a soft-bristled brush, such as a toothbrush and/or pipe brush. Scrub device below water line to prevent aerosolization of contaminants.
c. Following scrub, inspect device for visible soil residue.
3. Rinse
a. Remove the device from the soap solution and thoroughly rinse under flowing tap water for a minimum of 30 seconds.
b. Allow the device to dry.
*New pessaries are powdered with food-grade powder.
EVALUATING THE FIT:
Patient should be able to stand and bear down slightly without dislodging Pessary. Expulsion or turning of Pessary may indicate that Pessary size is too small. Therefore, the next larger size needs to be evaluated. Patient should be able to comfortably sit, stand and bend with Pessary in place. Pelvic discomfort during these activities may indicate that the Pessary size is too large and that a smaller size should be evaluated. Patient should be able to urinate without difficulty with Pessary in place, otherwise it may indicate an improp- erly-positioned or incorrectly-sized Pessary. If Pessary is too tight, it may cause discomfort or vaginal wall tissue damage; too loose, it may be easily expelled.
REMOVE, CLEAN AND SUGGESTED FOLLOW-UP:
1. To remove Pessary, carefully move Pessary forward toward introitus. When Pessary is within reach, compress Pessary and carefully withdraw.
2. Pessary should be removed for cleaning every day or every other day. Pessary can be cleaned with mild soap and warm water.
3. Discuss with patient the importance of following instructions and the expected length of time for Pessary usage.
4. Schedule follow-up visits to fit the needs of patient.
5. Instruct patient to immediately report any of the following: Discomfort, Spontaneous explusion, Any changes in color, amount, consistency, or odor of vaginal discharge, Difficulty with elimination

